

Designed by: Soumyadeep Mukhopadhyay
Site owned by: Bhaskar Sengupta, Heriot-Watt University, Edinburgh
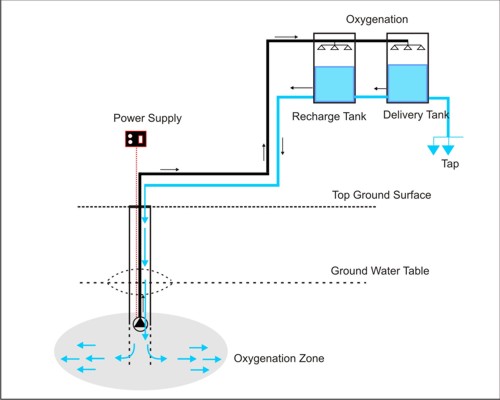
In the in-situ treatment method, the aerated tube well water is stored in feed water tanks and released back into the aquifers through the tube well by opening a valve in a pipe connecting the water tank to the tube well pipe under the pump head. The dissolved oxygen in aerated water oxidizes arsenite to less-mobile arsenate, the ferrous iron to ferric iron and Manganese(II) to Manganese(III), followed by adsorption of arsenate on Fe(III) and manganese(III) resulting in a reduction of the arsenic content in tube well water. Oxidation is further enhanced biologically by bacteria living in the subsurface and is termed bioremediation process. Because of the input of oxygen, the redox potential of the water is increased. A number of different physical, chemical and biological processes are intensified in the surrounding area of the well screen section, the so called oxidation zone. The alternate operation of the wells for delivering groundwater in the tank top and infiltration of the oxygen rich water into the aquifer induces alternating oxidation and adsorption periods on the surface of the solid material in the aquifer.
The process of in-situ oxidation of groundwater virtually transfers the oxidation and filtration process of the conventional above ground water treatment plants into the aquifer. The underground aquifer is used as a natural biochemical reactor.
The in situ method is a very cost effective and eco-friendly process for arsenic removal. The greatest advantage of this process is there is no need for sludge handling. The arsenic which is trapped into the sand along with the iron flocs constitute a infinitesimal volume of the total volume being handled and hence pose very little environmental threat in its adsorbed form. The whole mass remains down below unlike other processes where there is extra cost of sludge handling and messy disposal problem. The process is chemical free, simple and easy to handle. There is no restriction to the volume it can handle as long as proper time is allowed for the oxygen rich impregnated water to create the adequate oxidizing zone in the deep aquifer. It is also quite flexible with respect to the raw water quality as the efficient coefficient could be varied depending on the quality of the raw water. It involves low capital cost and minimum operating cost . The results obtained in the test site is quite promising as the process is able to reduce the arsenic content from 100-250 mg/l to permissible limit. It is ideal for a rural set up where people really cannot afford to pay a substantial amount for water supply. The only disadvantage is that it takes some time for the whole system to stabilise because of the slow kinetics of the oxidation process. However, once stabilised, it remains steady for years to come.
On the other hand, uncalculated amount of oxidation of the aquifer can really mess up the system resulting in As and Fe precipitation rather than adsorption (resulting in As release in later date). Also, the abrupt change in redox potential and huge oxidation may destroy the existing bacterial population, making the whole process unstable and ineffective.
WORKING PRINCIPLE
Name of Plant
|
Starting of Operation
|
Delivery of Water
|
Merudandi, Basirhat,
North 24 Parganas, WB
|
12th June, 2008
|
18th august, 2008
|
Purbapara, Basirhat
North 24 Parganas, WB
|
12th June, 2008
|
5th August, 2008
|
Rangapur, Nilgunj
North 24 Parganas, WB
|
10th October, 2008
|
1st December, 2008
|
Gotra, Ghetugachi, Chakdah, Nadia, WB
|
20th October, 2008
|
16th December, 2008
|
Tepul, Gobardanga
North 24 Parganas, WB
|
3rd October, 2008
|
4th December, 2008
|
Naserkul, Ranaghat,
Nadia, WB
|
10th November, 2008
|
15th January, 2009
|
Plant
|
Capacity
|
Initial Arsenic conc (ppb)
|
Final Arsenic conc (ppb)
|
Initial Iron conc (ppb)
|
Final Iron conc (ppb)
|
Merudandi, Basirhat
|
3000 lt/cycle
|
282
|
10-50
|
3326
|
< 200
|
Naihati Purbapara, Basirhat
|
3000 lt/cycle
|
175
|
10-50
|
1573
|
< 200
|
Tepul, Gobardanga
|
4000 lt/cycle
|
158
|
10-50
|
2936
|
174
|
Ghetugachi, Chakdah
|
3000 lt/cycle
|
206.5
|
10-50
|
2077
|
< 200
|
Naserkul, Ranaghat
|
3000 lt/cycle
|
187
|
10-50
|
3225
|
177
|
Max Permissible Limit:
Arsenic: 10 ppb or 10 micrograms per liter (USEPA/WHO)
Iron: 0.3 mg per L or 300 ppb(USEPA)
"On January 22, 2001 EPA adopted a new standard for arsenic in drinking water at 10 parts per billion (ppb), replacing the old standard of 50 ppb. The rule became effective on February 22, 2002. The date by which systems must comply with the new 10 ppb standard is January 23, 2006."
Source: http://water.epa.gov/lawsregs/rulesregs/sdwa/arsenic/regulations.cfm
SITE DETAILS
1. Most effective & long lasting technology available presently. With regular treatment, the arsenic level will steadily remain >2 ppb. The currently operating SAR plants have maintained the water quality for the past three and half years.
2. It is also the easiest technology around. Every plumber & electrician in the village can do the operation & maintenance once they are given a simple training.
3. The SUSTAINABLE way of treating arsenic contaminated water. This process not only removes arsenic & iron from the water pumped out through the system, but also treats the contaminated aquifer.
4. Easy Operation & Maintenance: The whole system is very cheap to operate & maintain.
5. More there is iron in water, more effective the process is. The arsenic gets adsorbed in the soil particles coated with Fe(III) is not released.
6. Waste disposal is not a problem. During operational phase, nearly ZERO waste is generated since iron, arsenic & other impurities such as Mn, nitrates, nitrites, etc gets adsorbed in the soil particles within the aquifer itself.
7. Large supply of Arsenic free water can be obtained by running the treatment cycle for more than once per day.
ADVANTAGES
1. The ground water must contain a high concentration of iron. (As:Fe = 1: 10 will be excellent. If that conc ratio is present, then this method will work whatever may be th level of arsenic)
2. The spray system must work properly & efficiently.
3. Small handpumps can not be used for this process. Submersible pumps working through electrical power is a necessity to achieve the desired result.
LIMITATIONS